Introduction
Prostate cancer remains one of the most significant health challenges affecting men worldwide. Traditionally, non-metastatic castration-resistant prostate cancer (nmCRPC) has posed a complex therapeutic dilemma, as the effectiveness of conventional androgen deprivation therapies (ADT) tends to wane over time. In this challenging landscape, Nubeqa (darolutamide) emerged as a breakthrough therapy during clinical trials, and its real-world performance has further cemented its role as a transformative treatment option. Today, Nubeqa is celebrated not only for its success in clinical trials but also for its robust performance in everyday clinical settings. This article explores the journey of Nubeqa beyond clinical trials, focusing on its active ingredient, mechanism of action, clinical efficacy, safety profile, cost and accessibility, market performance with a special emphasis on Nubeqa sales, and its promising future in prostate cancer care.
For more in-depth insights on Nubeqa’s development and future potential, download the full report @ Nubeqa Market Report.
What is Nubeqa?
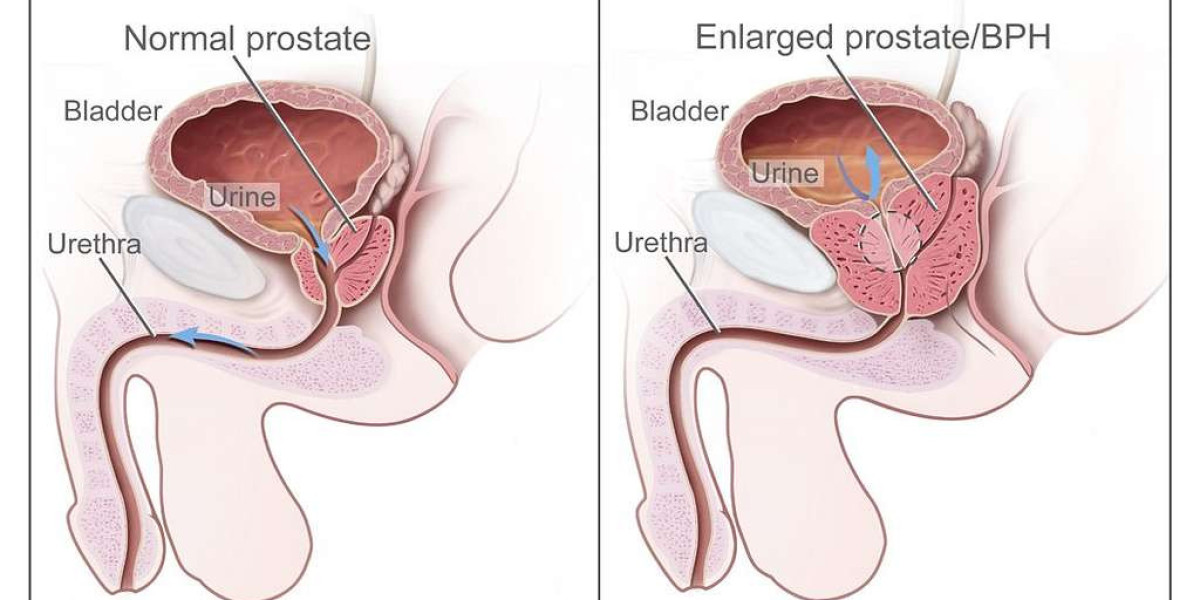
Nubeqa is an innovative oral androgen receptor inhibitor designed specifically for the treatment of nmCRPC. Its active ingredient, darolutamide, works by blocking the interaction between androgens and cancer cells, effectively slowing the progression of the disease. Unlike traditional therapies that can become less effective over time, Nubeqa has demonstrated the ability to delay the onset of metastasis, providing patients with a longer period of metastasis-free survival. The drug received FDA approval after the landmark ARAMIS trial, a pivotal study that underscored its clinical benefits and favorable safety profile. Today, Nubeqa stands as a cornerstone in modern prostate cancer management, particularly valued for its precision and improved tolerability, making it a preferred treatment option among oncologists and patients alike.
Nubeqa Mechanism of Action (MOA)
The success of Nubeqa in both clinical trials and real-world settings is largely attributed to its well-defined mechanism of action. At its core, Nubeqa’s mechanism involves a high-affinity binding to androgen receptors (AR), which in turn prevents the stimulation of cancer cells by testosterone. This targeted approach effectively disrupts the gene expression pathways that are critical for tumor survival and proliferation. What sets Nubeqa apart from earlier androgen receptor inhibitors is its molecular design; it minimizes the penetration of the blood-brain barrier, which translates into a significantly lower risk of neurological side effects such as seizures or cognitive impairment. This distinct characteristic has garnered attention not only in clinical trial data but also in real-world applications, where maintaining patients’ functional status is paramount. The precision inherent in Nubeqa’s mechanism of action is a key factor that underpins its success and continues to influence the evolving landscape of prostate cancer therapy.
Clinical Efficacy and Safety
Nubeqa’s real-world performance is a testament to its clinical efficacy and safety, as initially demonstrated in the ARAMIS trial. The trial’s outcomes revealed that Nubeqa extended metastasis-free survival by nearly two years compared to placebo, a breakthrough in the management of nmCRPC. Patients enrolled in the study also experienced delayed pain progression and maintained higher quality-of-life scores, even as the disease progressed. Importantly, the drug’s safety profile has proven favorable; the most common side effects—such as fatigue and hypertension—were generally mild and manageable. The lower discontinuation rates associated with Nubeqa, compared to other androgen receptor inhibitors, have been particularly notable in real-world settings, where long-term treatment adherence is crucial. The combination of robust clinical trial results and consistent real-world evidence has not only led to widespread regulatory approvals but has also contributed to Nubeqa’s reputation as a treatment that genuinely improves patient outcomes over the long term.
For more detailed insights and the latest updates on Nubeqa, visit the Nubeqa Market update.
Nubeqa Cost and Accessibility
While innovative therapies often come with significant price tags, Nubeqa’s overall cost-effectiveness is underscored by its ability to delay costly metastatic interventions. This delay not only translates to improved patient outcomes but also alleviates the financial burden on healthcare systems over time. Expanding insurance coverage across major markets such as the United States, the EU5, and Japan has been instrumental in making Nubeqa accessible to a broader patient population. Additionally, Bayer and Orion Corporation have initiated patient assistance programs that further reduce out-of-pocket expenses, ensuring that financial constraints do not impede access to this vital therapy. The strategic focus on cost and accessibility has enabled Nubeqa to transition seamlessly from clinical trial success to real-world impact, where economic considerations play a critical role in treatment adoption.
Nubeqa Sales and Market Performance
The robust performance of Nubeqa in clinical settings has been mirrored by its impressive market performance, a key indicator of its real-world success. Nubeqa sales have surged since its launch, reflecting not only the high unmet need in the treatment of nmCRPC but also the confidence that healthcare providers and patients place in its efficacy and tolerability. Analysts predict that Nubeqa sales will continue to grow robustly over the next decade, driven by increasing diagnosis rates, favorable reimbursement policies, and the drug’s expanding footprint in emerging markets. The United States leads in revenue generation due to both high incidence rates of prostate cancer and progressive insurance frameworks, while markets in Europe and Japan have shown significant promise as awareness and adoption rates rise. Furthermore, the sustained momentum in Nubeqa sales is underpinned by ongoing efforts to integrate the therapy into broader treatment guidelines, ensuring that its use extends well beyond the confines of clinical trials. The impressive performance in Nubeqa sales not only validates the drug’s clinical benefits but also highlights its potential to remain a dominant force in prostate cancer care.
For further insights and detailed research on this breakthrough treatment, visit Nubeqa Insights.
Future Outlook and Innovations
Looking ahead, the future of Nubeqa appears as promising as its present, with multiple avenues of innovation and expansion on the horizon. Ongoing clinical studies are exploring the potential of Nubeqa in treating metastatic hormone-sensitive prostate cancer (mHSPC), with early data suggesting significant survival benefits. There is also a growing interest in investigating combination therapies that pair Nubeqa with chemotherapy or PARP inhibitors to combat resistant forms of the disease. These efforts are indicative of a broader strategy to extend the therapeutic applications of Nubeqa, ensuring that its benefits reach an even wider patient population. Additionally, as the market evolves and patents approach expiration, the introduction of biosimilar competition is anticipated. However, the well-established efficacy and safety profile of Nubeqa, as demonstrated through both clinical trials and real-world data, is expected to maintain its market relevance. Advances in biomarker-driven care, such as AR-V7 testing, are likely to further personalize treatment protocols, thereby optimizing outcomes for patients identified as high-risk. With a continued focus on innovation, Nubeqa is poised to remain at the forefront of prostate cancer therapy, constantly evolving to meet the demands of modern oncology practice.
For additional insights on Nubeqa’s transformative potential, please download the full Nubeqa report.
Conclusion
Nubeqa represents a significant leap forward in the treatment of prostate cancer, bridging the gap between clinical trial success and real-world performance. Its active ingredient, darolutamide, has revolutionized the approach to nmCRPC by delivering targeted, precision-based therapy with a superior safety profile. The favorable results from Nubeqa Clinical Trials have been seamlessly translated into everyday clinical practice, where the drug continues to deliver extended metastasis-free survival and improved quality of life for patients. Robust Nubeqa Approvals across global markets have facilitated its widespread adoption, further bolstered by strong insurance coverage and patient assistance programs that enhance its cost-effectiveness and accessibility.
The sustained momentum in Nubeqa sales, a critical marker of its market performance, reflects the confidence of both healthcare professionals and patients in its therapeutic benefits. As Nubeqa sales continue to drive its market presence, the ongoing pursuit of additional indications and combination therapies signals an exciting new chapter in prostate cancer management. In an era where precision medicine is reshaping the landscape of oncology, Nubeqa stands as a testament to the power of innovative science and its ability to transform patient care. With a promising future enriched by continuous clinical innovation and expanding market reach, Nubeqa is well-positioned to remain an indispensable asset in the oncologist’s arsenal, turning prostate cancer from a formidable adversary into a manageable chronic condition.
In summary, the real-world success of Nubeqa extends far beyond the confines of clinical trials. It encapsulates a journey of innovation, precision, and sustained market performance that has redefined the standard of care for nmCRPC. As we look forward, the continued evolution of Nubeqa is set to further enhance its role in prostate cancer therapy, offering renewed hope and tangible benefits to patients around the globe.
For those looking to explore this breakthrough treatment more, download the full Nubeqa Insights Report.
Read More
- Prostate Cancer Market Insight, Epidemiology and Market Forecast
- Metastatic Prostate Cancer Market Insight, Epidemiology And Market Forecast
- Non-metastatic Prostate Cancer (nmPC) - Market Insight, Epidemiology and Market Forecast
- Metastatic Castration-Sensitive Prostate Cancer (mCSPC) - Market Insight, Epidemiology And Market Forecast
- Metastatic Castration-Resistant Prostate Cancer Market Insight, Epidemiology And Market Forecast
About DelveInsight
DelveInsight is a leading business Healthcare consultancy and market research firm specializing in life sciences. It assists pharmaceutical companies by offering comprehensive, end-to-end solutions to improve performance. Access all our healthcare and pharmaceutical market Competitive Intelligence Solutions.