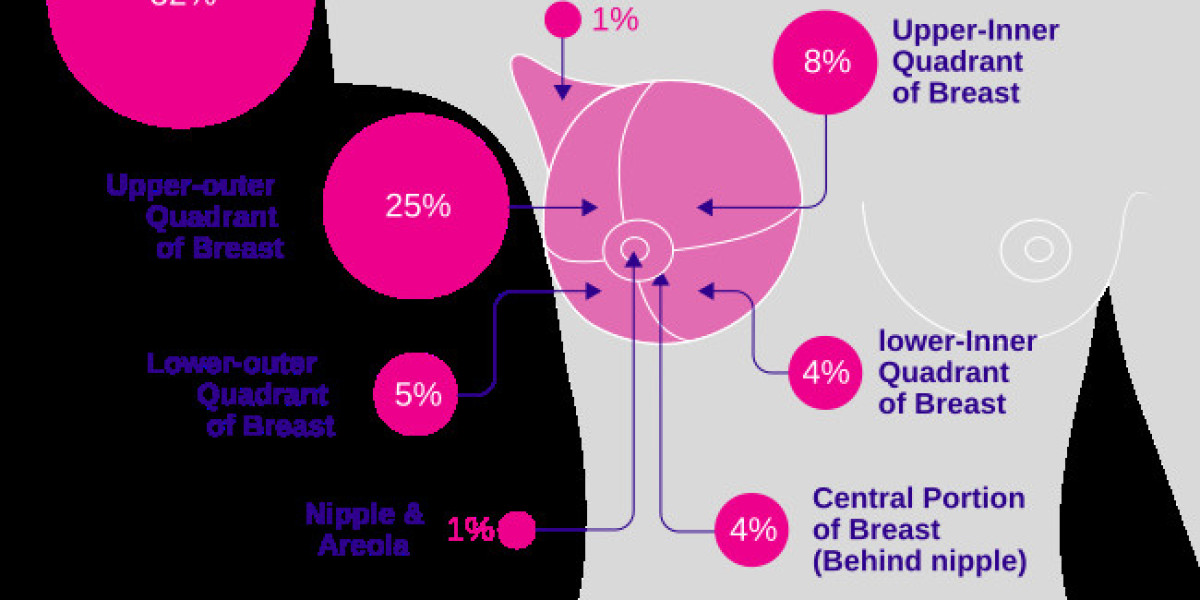
Introduction
The landscape of breast cancer management has undergone a revolutionary transformation in recent years. Advances in precision medicine have paved the way for treatments that not only target the disease more effectively but also offer long-term benefits to patients. Among these innovations, Lynparza stands out as a beacon of hope. Originally developed for patients with ER-positive, HER2-negative breast cancer, Lynparza has forged a clinical legacy built on scientific precision and personalized care. Its long-term benefits have reshaped treatment paradigms, reducing recurrence rates and improving quality of life for many. As research continues to unfold, Lynparza’s impact grows, solidifying its position as a cornerstone in modern oncology.
For more in-depth insights on Lynparza’s development and future potential, download the full report @ Lynparza Market Report.
What is Lynparza?
Lynparza is an oral PARP inhibitor whose active ingredient, olaparib, has redefined the approach to treating breast cancer. Initially approved by the FDA for metastatic ER+ HER2- breast cancer in patients carrying germline BRCA mutations, its indications have since expanded. Lynparza is now used not only in the metastatic setting but also as adjuvant therapy in high-risk early-stage breast cancer. The therapy’s design emphasizes personalized care, leveraging companion diagnostic tests to ensure that only patients who are most likely to benefit receive the treatment. By targeting the genetic characteristics of the tumor, Lynparza exemplifies how precision oncology can lead to improved long-term outcomes while minimizing unnecessary side effects.
Lynparza Mechanism of Action (MOA)
At the heart of Lynparza’s success lies its innovative mechanism of action. Lynparza works through the principle of synthetic lethality—a process that exploits the inherent weaknesses in cancer cells’ DNA repair pathways. By inhibiting PARP enzymes, Lynparza effectively blocks the repair of single-strand DNA breaks. In cells with compromised repair mechanisms, particularly those with BRCA mutations or homologous recombination deficiency (HRD), this inhibition results in the accumulation of DNA damage. The failure to repair this damage eventually leads to lethal double-strand breaks that cancer cells cannot overcome, thereby curtailing tumor progression.
The significance of Lynparza’s mechanism is underscored by its ability to specifically target tumor cells while sparing normal, healthy cells. This selective targeting minimizes collateral damage and underscores the treatment’s favorable safety profile. Lynparza’s mechanism of action not only represents a leap forward in therapeutic strategy but also highlights the critical role of genetic testing in modern cancer care. Through its well-defined pathway, Lynparza active ingredient, olaparib, provides a robust example of how targeted interventions can offer durable clinical benefits over the long term.
For more detailed insights and the latest updates on Lynparza, visit the Lynparza Market update.
Clinical Efficacy and Safety
Lynparza has been at the forefront of clinical innovation in breast cancer management, a fact that is evident from its robust performance in clinical trials. In extensive studies, Lynparza has demonstrated significant efficacy both as a maintenance therapy following chemotherapy and as an adjuvant treatment for high-risk patients. Clinical trials have shown that the use of Lynparza can substantially delay disease recurrence, offering patients a critical extension in progression-free survival. This benefit is especially important in ER+ HER2- breast cancers, where the need for effective long-term management is paramount.
Safety has always been a priority in Lynparza’s clinical development. While some patients may experience manageable side effects such as fatigue or anemia, these adverse effects are generally well controlled through dose adjustments and careful monitoring. The favorable safety profile of Lynparza is bolstered by ongoing research and the routine integration of HRD testing into patient evaluation. Moreover, the ease of oral administration adds to the therapy’s appeal, providing a convenient alternative to more invasive treatment methods. As ongoing Lynparza Clinical Trials continue to validate its long-term benefits, the treatment’s clinical efficacy remains a testament to its transformative role in oncology.
Lynparza Cost and Accessibility
The adoption of Lynparza has been accompanied by a broader conversation about cost and accessibility in modern cancer care. While the price tag associated with Lynparza reflects the high level of innovation and research investment involved, its long-term benefits in reducing hospitalizations, managing disease progression, and improving patient outcomes present a compelling cost-benefit proposition. Insurance coverage for Lynparza has expanded significantly, especially for patients with confirmed BRCA mutations. Furthermore, various manufacturer assistance programs have been established to help mitigate out-of-pocket expenses, ensuring that more patients can access this breakthrough therapy.
Efforts to promote universal HRD testing are also gaining momentum, which could further improve the timely identification of eligible patients. By integrating advanced diagnostic tools with Lynparza therapy, healthcare providers are better equipped to tailor treatments that maximize efficacy while considering cost constraints. In this context, the discussion of Lynparza Approvals is particularly relevant, as the expansion of its indications through rigorous regulatory review continues to bolster confidence among clinicians and patients alike.
For further insights and detailed research on this breakthrough treatment, visit Lynparza Insights.
Lynparza Sales and Market Performance
The commercial success of Lynparza is a critical aspect of its overall legacy. Its pioneering role in targeting ER+ HER2- breast cancer has not only improved clinical outcomes but also driven significant market growth. Lynparza sales have been a key indicator of the therapy’s acceptance in the oncology community. With increased genetic testing, expanding FDA approvals, and strategic partnerships between AstraZeneca and Merck, Lynparza has carved out a dominant position in the competitive PARP inhibitor market. In discussions about market performance, Lynparza sales remain a recurring and significant topic, as they reflect both the therapy’s clinical impact and its economic viability.
Market forecasts predict continued robust growth for Lynparza sales in the coming years, underpinned by its established efficacy and the ongoing expansion of its approved uses. As new data from Lynparza Clinical Trials further validate its benefits, the momentum behind Lynparza sales is expected to increase. This commercial strength is indicative of broader trends in personalized medicine, where therapies are not only clinically effective but also economically sustainable. The persistent mention of Lynparza sales in the literature and industry reports underscores its importance in shaping future therapeutic strategies.
Future Outlook and Innovations
The future of Lynparza is both promising and dynamic. Ongoing research is exploring the potential of combining Lynparza with other therapeutic agents, such as immunotherapy and CDK4/6 inhibitors. These combination strategies are aimed at overcoming resistance mechanisms in advanced cases of breast cancer and further enhancing the long-term benefits of the treatment. Moreover, technological advancements in liquid biopsy and HRD testing could expand the reach of Lynparza, allowing for even earlier and more accurate identification of candidates who may benefit from its use.
Beyond its current indications, Lynparza is being investigated in other HRD-positive cancers, including gastric and bladder cancers. These studies could pave the way for additional Lynparza Approvals, further extending its clinical legacy and demonstrating its versatility across multiple oncological settings. As more data from ongoing trials become available, the role of Lynparza in redefining cancer care will likely expand, setting new benchmarks in precision oncology. The integration of these innovations promises not only to enhance patient outcomes but also to consolidate the long-term clinical benefits that Lynparza offers.
For additional insights on Lynparza’s transformative potential, please download the full Lynparza report.
Conclusion
Lynparza’s clinical legacy is firmly rooted in its ability to transform breast cancer management through precision, personalized care. From its innovative mechanism of action to its impressive performance in clinical trials, Lynparza represents a new era in oncology where treatments are tailored to the genetic profile of the disease. The active ingredient olaparib has proven to be a powerful tool in targeting the DNA repair vulnerabilities of cancer cells, offering both immediate and long-term benefits to patients.
The economic impact of Lynparza, reflected in its strong market performance and notable Lynparza sales, further underscores its significance in the current therapeutic landscape. With expanded indications and ongoing studies, Lynparza is well positioned to remain a leader in the realm of precision oncology. As advancements in diagnostic testing and combination therapies continue to evolve, the future of Lynparza appears bright, promising even greater strides in the fight against breast cancer.
In summary, Lynparza’s journey from an innovative concept to a clinically validated therapy is a testament to the progress made in the field of personalized medicine. Its clinical trials have consistently demonstrated that targeted interventions, when aligned with genetic insights, can yield durable and meaningful benefits for patients. With each new Lynparza Approval and the ongoing success of Lynparza sales, the therapy not only reaffirms its status as a groundbreaking treatment but also sets the stage for future innovations that will continue to shape the long-term management of breast cancer.
The enduring legacy of Lynparza is a reminder of the power of precision medicine—a future where every patient receives treatment that is as unique as their genetic makeup. As we look ahead, Lynparza’s contributions to the field will undoubtedly inspire further breakthroughs, ultimately transforming the lives of countless individuals affected by breast cancer.
For those looking to explore this breakthrough treatment more, download the full Lynparza Insights Report.
Read More
- Lynparza - API Insight
- Metastatic HR+/HER2− Breast Cancer - Market Insights, Epidemiology, and Market Forecast
- Metastatic HER2 positive Breast Cancer - Market Insight, Epidemiology And Market Forecast
About DelveInsight
DelveInsight is a leading business Healthcare consultancy and market research firm specializing in life sciences. It assists pharmaceutical companies by offering comprehensive, end-to-end solutions to improve performance. Access all our healthcare and pharmaceutical market Competitive Intelligence Solutions.