OPZELURA (ruxolitinib) cream has emerged as a revolutionary treatment for atopic dermatitis, providing patients with a non-steroidal option that bridges advanced scientific innovation with market success. By targeting the underlying inflammatory pathways, this breakthrough topical therapy offers hope to millions who suffer from the chronic discomfort of eczema. The rapid growth in OPZELURA sales underscores both its clinical efficacy and the increasing demand from healthcare providers and patients alike.
For more in-depth insights on OPZELURA’s development and future potential, download the full report @ OPZELURA Market Report
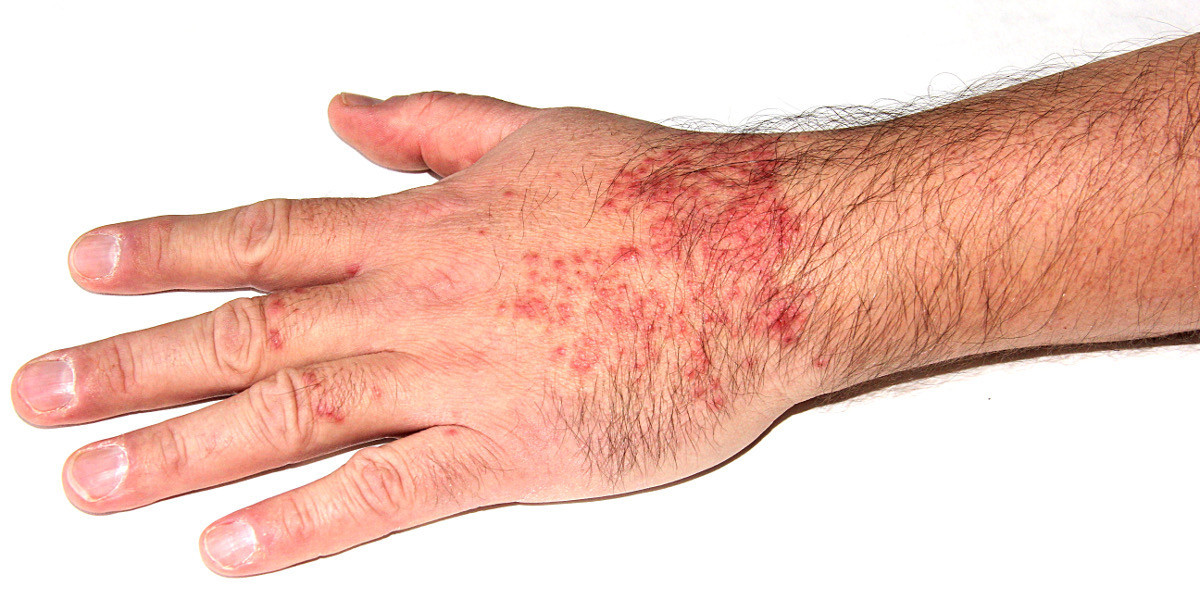
Understanding Atopic Dermatitis
Atopic dermatitis (AD), commonly known as eczema, is a chronic inflammatory skin condition that affects individuals worldwide. This condition is characterized by dry, scaly skin, intense itching, red and inflamed patches, and a compromised skin barrier that leads to frequent infections. The multifactorial nature of AD—stemming from genetic, environmental, and immune system factors—means that its symptoms can range from mild irritation to severe, debilitating outbreaks. For many, the struggle with AD extends far beyond physical discomfort, impacting sleep, self-esteem, and overall quality of life. Traditional treatments such as emollients, corticosteroids, and calcineurin inhibitors, while beneficial, have their limitations. These therapies often come with potential side effects, particularly when used long-term, necessitating the need for innovative alternatives.
Introduction to OPZELURA (Ruxolitinib)
OPZELURA represents a significant milestone in the treatment of atopic dermatitis. Developed by Incyte Corporation, this topical formulation contains ruxolitinib—the OPZELURA active ingredient—which functions as a Janus kinase (JAK) inhibitor. Approved by the U.S. Food and Drug Administration (FDA) in September 2021, OPZELURA is indicated for patients aged 12 and older with mild to moderate AD who are not immunocompromised. The FDA approval of OPZELURA stands as a testament to its safety and efficacy, positioning it as a preferred non-steroidal alternative for patients seeking relief without the complications often associated with corticosteroid use.
For more detailed insights and the latest updates on OPZELURA, visit the OPZELURA Market update
Mechanism of Action (MOA)
The success of OPZELURA is largely rooted in its targeted approach to inflammation. OPZELURA’s Mechanism of Action involves inhibiting the JAK1 and JAK2 pathways, crucial components of the JAK-STAT signaling cascade that mediate inflammatory and immune responses. By directly blocking these pathways, ruxolitinib reduces the production and activity of cytokines responsible for triggering the symptoms of atopic dermatitis. This precise inhibition leads to a rapid decrease in inflammation, itch, and skin barrier dysfunction. The efficacy of this mechanism is clearly demonstrated in clinical settings, where patients experience marked improvements in skin clarity and symptom relief shortly after application.
Clinical Efficacy of OPZELURA
Clinical trials have played a pivotal role in establishing the effectiveness of OPZELURA. Notably, the Phase 3 TRuE-AD trials (TRuE-AD1 and TRuE-AD2) involved over 1,200 patients with mild to moderate atopic dermatitis. These studies revealed that a significant proportion of patients achieved clear or nearly clear skin, as measured by standard dermatological assessment tools. Additionally, many participants reported a rapid reduction in itch severity—some noticing relief as early as 24 to 48 hours after the first application.
Beyond the controlled environment of clinical trials, real-world evidence further supports the sustained benefits of OPZELURA. Patients using the cream have shown high adherence rates, improved sleep patterns, and an overall enhancement in quality of life. These positive outcomes are not only reflected in clinical data but also in robust OPZELURA sales, which continue to climb as more dermatologists and patients recognize its benefits.
The impressive results from OPZELURA Clinical Trials have reinforced its position as a front-runner in topical AD therapy. Its success in these trials, coupled with its strong market performance, illustrates the ideal balance of scientific innovation and practical application—a balance that has propelled OPZELURA Approvals in multiple regions, ensuring its availability to a wider patient population.
For further insights and detailed research on this breakthrough treatment, visit OPZELURA insights
Safety Profile and Side Effects
While OPZELURA is celebrated for its clinical benefits, it is important to consider its safety profile. The cream is generally well tolerated, with most patients experiencing only minor side effects. Reported adverse effects include mild application site reactions such as redness, burning, or stinging. Some individuals may also experience common cold symptoms or headaches. Due to the localized application and minimal systemic absorption, the risk of systemic side effects—such as significant blood count changes or increased infection risk—is considerably lower compared to oral JAK inhibitors. Nonetheless, patients with active infections or a history of cardiovascular and thromboembolic issues should exercise caution and consult their healthcare providers before beginning treatment.
Comparisons with Other Treatments
In the realm of atopic dermatitis management, OPZELURA distinguishes itself from traditional treatments. Compared to topical corticosteroids, OPZELURA offers comparable, if not superior, efficacy in reducing inflammation and itch without the risk of skin thinning or hormonal axis suppression. Similarly, when set against calcineurin inhibitors like tacrolimus and pimecrolimus, OPZELURA demonstrates faster symptom relief and a more favorable tolerability profile, avoiding the burning sensation sometimes associated with these alternatives.
For patients requiring treatment for localized AD, OPZELURA presents a targeted, topical option that stands apart from systemic therapies such as dupilumab or baricitinib. While systemic treatments may be necessary for severe cases, OPZELURA offers a more accessible and safer alternative for individuals with mild to moderate symptoms. This advantage is further highlighted by its growing OPZELURA sales, which reflect a broader acceptance and preference in the market over older treatment regimens.
For additional insights on OPZELURA’s transformative potential, please download the full OPZELURA report
Patient Considerations
OPZELURA is particularly beneficial for patients who have not found sufficient relief from conventional treatments or those who wish to avoid the long-term risks associated with corticosteroids. It is recommended for individuals aged 12 and above who experience frequent flare-ups and are in need of a sustained, non-steroidal treatment approach. On the other hand, patients with active infections or a history of serious cardiovascular or thromboembolic conditions should consult their healthcare providers prior to using OPZELURA. Furthermore, its use in pregnant or breastfeeding women should be carefully evaluated and monitored to ensure both maternal and fetal safety.
Future Perspectives
The journey of OPZELURA in the treatment landscape of atopic dermatitis is just beginning. Ongoing research is exploring its potential applications beyond eczema, including its use in other inflammatory skin disorders such as vitiligo, psoriasis, and alopecia areata. Advances in the formulation and delivery of JAK inhibitors promise to enhance both the safety and efficacy of topical treatments, potentially broadening the scope of conditions that can be managed with OPZELURA. As the scientific community continues to unravel the intricacies of OPZELURA’s Mechanism of Action, future studies are poised to optimize dosing strategies, minimize side effects, and extend its benefits to even more patient populations. With robust OPZELURA sales reflecting market confidence, the horizon looks promising for further innovations in topical dermatological therapies.
For those looking to explore more about this breakthrough treatment, download the full OPZELURA Insights Report
Conclusion
OPZELURA (ruxolitinib) cream is transforming the management of atopic dermatitis by combining cutting-edge science with tangible clinical success. Its targeted approach—rooted in the inhibition of the JAK1 and JAK2 pathways—provides rapid and effective relief from the symptoms of AD while offering a safer, non-steroidal alternative to conventional treatments. The compelling results from OPZELURA Clinical Trials, the favorable safety profile, and the continuous rise in OPZELURA sales underscore its pivotal role in both the current and future landscape of dermatological care. As research continues to expand its applications, OPZELURA Approvals across different regions are a promising sign of its potential to revolutionize the treatment of inflammatory skin disorders globally.
Related Reports
- Atopic Dermatitis Market Insight, Epidemiology and Market Forecast
- Radiation Dermatitis Market Insight, Epidemiology and Market Forecast
- Seborrhoeic Dermatitis - Market Insight, Epidemiology and Market Forecast
About DelveInsight
DelveInsight is a leading business Healthcare consultancy and market research firm specializing in life sciences. It assists pharmaceutical companies by offering comprehensive, end-to-end solutions to improve their performance. Access all our healthcare and pharmaceutical market Competitive Intelligence Solutions.