Summary
- UK Quality Management Certification is vital for quality objectives and regulatory compliance in medical devices.
- Continuous improvement, with UK ISO 13485 Services and audits, enhances quality and ensures compliance.
- Optimal continuous improvement includes cultural and process balance, aligned leadership, data-driven decisions, and flexibility.
- Combining TQM, Six Sigma, Lean Thinking, or Theory of Constraints maximizes improvement effectiveness.
- Fostering a continuous improvement culture demands leadership commitment, employee empowerment, and training.
Short Description
In the dynamic and highly regulated medical device industry, embracing continuous improvement within quality management systems is key. By implementing best practices and adopting a proactive approach towards quality management, organizations can effectively enhance their systems to surpass industry benchmarks and deliver products that meet the highest standards of performance and safety.
Image: https://www.pexels.com/photo/women-colleagues-gathered-inside-conference-room-1181622/
Introduction
Quality management systems (QMS) are the backbone of ensuring quality and compliance in the medical device industry. A QMS outlines the processes, procedures, and responsibilities for achieving quality objectives across all stages of a medical device's lifecycle - from planning and design to manufacturing and commercialization. Integrating with other business operations like procurement and resource management, a robust QMS promotes best practices supported by UK Quality Management Certification.
A QMS assists the manufacturer in providing sufficient employee training, ensuring compliance with customer requirements, adhering to legal and regulatory mandates, and monitoring key performance indicators for business execution, with assistance from UK ISO 13485 Services. If any inefficiencies emerge in these areas, corrective measures are taken through established processes, followed by effectiveness assessments to confirm resolution. This ensures that any issues are promptly dealt with.
Significance of Continuous Improvement in UK Quality Management Certification
The evolution of quality standards, such as ISO 9001, has increasingly emphasized customer satisfaction and continuous improvement. In more recent iterations like ISO 9001:2000, the focus has shifted from quality assurance to enhancing customer satisfaction. In the context of medical device QMS, continuous improvement enables manufacturers to:
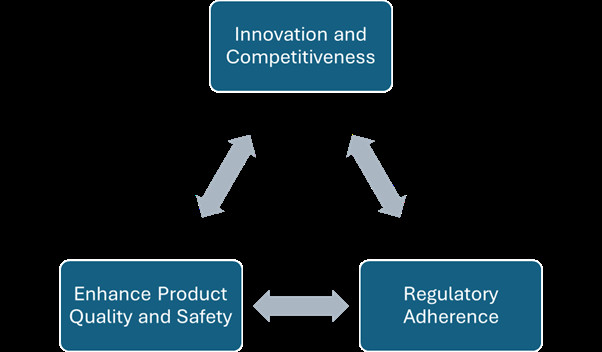
- Innovation and Competitiveness: Continuously improving processes by adhering to UK ISO 13485 Services can lead to cost savings, increased efficiency, and the development of innovative solutions, giving manufacturers a competitive edge.
- Regulatory Adherence: Regulatory bodies, such as the FDA and UK MDR, emphasize the importance of continuous improvement in maintaining effective QMS.
- Enhance Product Quality and Safety: By continuously refining processes and addressing non-conformances, manufacturers can improve product quality, reduce risks, and ensure patient safety.
Optimal Approaches for Continuous Improvement in QMS
Continuous improvement is essential for maintaining the effectiveness and relevance of QMS in today's ever-changing business environment. By continuously refining the processes and practices of UK Quality Management Certification, organizations can increase efficiency, ensure customer satisfaction, and enhance product quality. Optimal approaches for continuous improvement in QMS encompass a range of strategies aimed at identifying areas for enhancement and implementing targeted solutions that include:
- Align with Regulatory Requirements: Continuous improvement initiatives with evolving regulatory requirements, guidance documents, and industry best practices to maintain compliance and incorporate necessary changes into the QMS.
- Balancing Cultural and Process Perspectives: Culture-focused approaches, such as Total Quality Management (TQM) and Lean Thinking, promote employee empowerment and shared knowledge, fostering a collective dedication to quality and betterment of QMS. Meanwhile, process-focused methods like Six Sigma and Theory of Constraints ensure the establishment of robust systems and centralized expertise to optimize processes.
- Leveraging Data-Driven Decision-Making: Effective continuous improvement hinges on data-driven decision-making and evidence-based problem-solving. Establishing robust data collection, analysis, and feedback mechanisms is crucial for identifying improvement opportunities in a QMS.
- Aligning Leadership Styles: Effective leadership is essential for the successful implementation of proper QMS strategies. Leadership styles should correspond with the chosen strategy's emphasis, whether cultural or process-oriented.
- Streamline Processes: Continuously evaluate and optimize processes to improve efficiency, reduce waste, and enhance product quality by employing techniques like value process mapping, lean methodologies, and stream mapping.
- Maintaining Flexibility and Adaptability: The medical device industry is always evolving, characterized by changing regulations, technological advancements, and shifting market demands. Continuous improvement approaches must be flexible and adaptable, enabling organizations to respond swiftly to changes and emerging challenges, while regular reviews and adjustments to improvement strategies are necessary to ensure their ongoing relevance and effectiveness.
- Embracing a Combination of Strategies: To maximize effectiveness, it's advisable to combine complementary continuous improvement strategies like TQM, Six Sigma, Lean Thinking, or Theory of Constraints. Popular combinations include Lean Thinking paired with Six Sigma, Six Sigma coupled with Theory of Constraints, and TQM integrated with Theory of Constraints.
Nurturing a Culture of Continuous Improvement
Cultivating an organizational culture that values quality and champions change is essential for driving effective continuous improvement within a medical device QMS. This mindset should be the core value of the organization, with leadership demonstrating a commitment to continuous improvement and empowering employees to contribute ideas and solutions. Regular training programs and ongoing education initiatives play a vital role in ensuring employees understand the importance of continuous improvement, enhance their problem-solving abilities, and stay informed about industry advancements, all supported by UK ISO 13485 Services. Furthermore, acknowledging and rewarding successful improvement initiatives can reinforce positive behaviors and inspire enthusiastic participation in continuous improvement endeavors across the organization.
CliniExperts, a distinguished regulatory consulting firm, offers comprehensive services aimed at guiding medical device manufacturers through the integration of UK Quality Management Certification, facilitating adherence to best practices. With a team of adept regulatory specialists and an in-depth understanding of industry standards, CliniExperts provides invaluable assistance at every stage of the QMS implementation journey. Moreover, CliniExperts' guidance ensures compliance with regulatory guidelines, streamlines documentation processes, and enhances overall adherence to stringent quality standards, thereby enabling manufacturers to uphold best practices.
Conclusion
In the fast-paced and strictly regulated medical device industry, continuous improvement is essential for maintaining an effective quality management system. Embracing best practices for ongoing enhancement allows manufacturers to optimize processes, improve product quality and safety, and ensure compliance with regulations. Through UK Quality Management Certification, medical device manufacturers are well-equipped to navigate complex regulatory requirements and elevate their quality management systems to meet international standards.
UK ISO 13485 Services allow medical device companies to thoroughly evaluate their processes to ensure compliance with international quality standards. By partnering with reputable auditing and management services like CliniExpert, manufacturers can position themselves as industry leaders, delivering medical devices that are safe, effective, and meet the highest standards of quality and regulatory compliance.



