Industry Analysis
Medical device testing market is projected to experience growth during the forecast period from 2022 to 2029. The market is expected to exhibit a CAGR of 10.8% from 2022 to 2029, and it is estimated to reach USD 8,423.14 million by 2029, up from USD 3,832.27 million in 2021.
Medical Device Testing Market report helps the manufacturer in finding out the effectiveness of the existing channels of distribution, advertising programs, or media, selling methods and the best way of distributing the goods to the eventual consumers. Taking up such market research report is all the time beneficial for any company whether it is a small scale or large scale, for marketing of products or services. It makes effortless for healthcare industry to visualize what is already available in the market, what market anticipates, the competitive environment, and what should be done to surpass the competitor.
Get a Free Sample of The Report: https://www.databridgemarketresearch.com/request-a-sample/?dbmr=global-medical-device-testing-market
Market Insights and Scope
Medical device testing involves verifying that a device can perform safely and effectively during use. During the development of new products, comprehensive design validation testing is conducted, which may include performance testing, toxicity and chemical analysis, and occasionally human factors or clinical testing. Ongoing quality assurance testing is typically more limited, often consisting of dimensional checks, some functional testing, and verification of packaging. There are various medical testing services available in the market, including inspection services, certification services, and others.
A meticulous study of the competitive landscape of the market has been given in the Medical Device Testing Market survey document along with the insights of the companies, financial status, trending developments, mergers, acquisitions and SWOT analysis. This market research will give a clear and specific idea to the readers about the overall market to take valuable decisions. The document focuses on the leading global industry players providing information such as company profiles, specifications, capacity, cost, revenue and contact information. An influential Data bridge market research report also offers comprehensive statistics on important aspects such as growth drivers, challenges and industry prospects.
Get full access to the report: https://www.databridgemarketresearch.com/reports/global-medical-device-testing-market
Industry Segmentation and Size
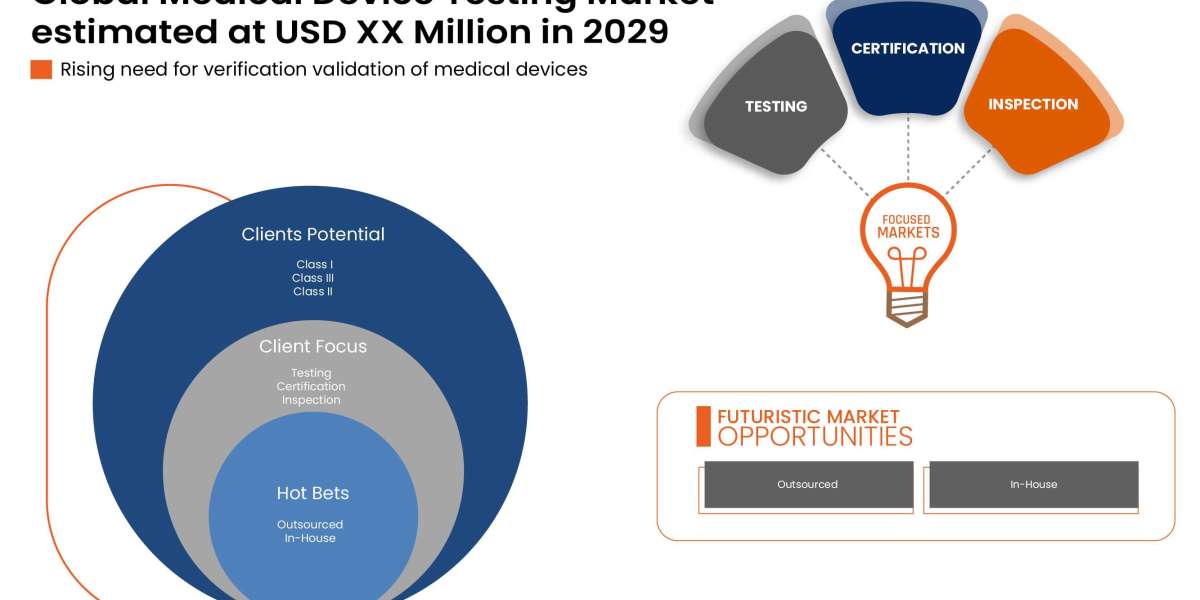
Global medical device testing market is segmented into service type, testing type, phase, sourcing type, device class and product. The growth amongst these segments will help you analyse meagre growth segments in the industries and provide the users with a valuable market overview and market insights to make strategic decisions to identify core market applications.
Service Type
- Testing services
- Inspection services
- Certification services
Testing Type
- Physical testing
- Chemical/biological testing
- Cybersecurity testing
- Microbiology and sterility testing
Phase
- Preclinical
- Clinical
Sourcing Type
- Outsourced
- In-house
Device Class
- Class I
- Class II
- Class III
Product
- Active implant medical device
- Active medical device
- Non-active medical device
- In-vitro diagnostics medical device
- Opthalmic medical device
- Orthopedic and dental medical device
- Vascular medical device
Market Country Level Analysis
The countries covered for this market are
- S., Canada, Mexico, Germany, France, U.K., Italy, Spain, Netherlands, Russia, Switzerland, Turkey, Belgium, Rest of Europe, China, Japan, India, South Korea, Australia, Singapore, Thailand, Malaysia, Indonesia, Philippines, Rest of Asia-Pacific, Brazil, Argentina, Rest of South America, South Africa, Saudi Arabia, UAE, Egypt, Israel, Rest of Middle East and Africa.
Industry Share Analysis
Some of the major players operating in the medical device testing market are
- Intertek Group plc, SGS SA, Bureau Veritas, TUV SUD, TUV Rheinland, Pace, Charles River Laboratories, Biomedical Device Labs, UL LLC, North American Science Associates, LLC, Medistri SA, WuXi AppTec, NSF, Labcorp, Eurofins Scientific, Nelson Laboratories, LLC- A Sotera Health company, Gateway Analytical, ITC ZLIN, Element Materials Technology, EndoLab Mechanical Engineering GmbH, Hohenstein, Medical Engineering Technologies Ltd., Bioneeds, Cigniti, Arbro Pharmaceuticals Private Limited Auriga Research Private Limited, Q Laboratories, IMR Test Labs among others.
Medical Device Testing Market Report Answers the Following Questions:
What is medical device testing and why is it important?
What are the different types of medical device testing services available in the market?
What is the process of design validation testing in medical device testing?
How does ongoing quality assurance testing differ from design validation testing?
What are the challenges faced in medical device testing?
What are the latest technological advancements in medical device testing?
How does regulatory compliance impact medical device testing?
What role do third-party testing and certification agencies play in the medical device testing market?
What is the outlook for the future of the medical device testing market?
What are the potential growth opportunities and challenges in the medical device testing market?
Browse Related Reports@
About Us:
Data Bridge Market Research set forth itself as an unconventional and neoteric Market research and consulting firm with an unparalleled level of resilience and integrated approaches. We are determined to unearth the best market opportunities and foster efficient information for your business to thrive in the market
Contact:
Data Bridge Market Research
Tel: +1-888-387-2818
Email: Sopan.gedam@databridgemarketresearch.com