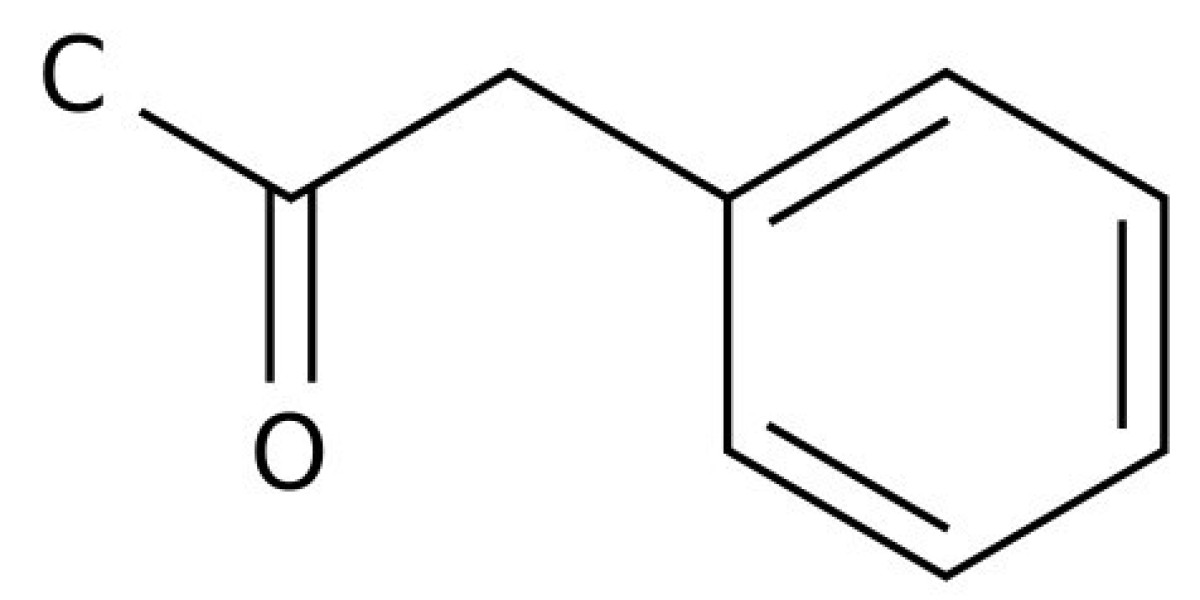
Method for preparing phenylacetone
The invention relates to a method for producing phenylacetone, which is an intermediate for obtaining a wide range of biologically active substances. In particular, on the basis of phenylacetone get vitamin B 12 , various indandionium rodenticides.
A known method of producing phenylacetone by reacting benzyl cyanide with ethyl acetate in sodium ethylate at a temperature of 45 ° C. The main disadvantage of this method is the low yield of the finished product 51-58 wt.% And a very large amount of water-salt waste, which is formed at the stage of neutralizing the excess sulfuric acid with alkali.
A known method of producing phenylacetone by reacting phenylacetic acid with acetic acid in the gas phase on various catalysts. So in US patent No. 2612524 as a catalyst used a mixture of magnesium oxide and magnesium hydroxide. In German patent No. 2758113, zirconia and / or thorium dioxide were used as a catalyst. A common disadvantage of these methods is primarily the low yield of the finished product - not higher than 61%, in addition, thorium dioxide is a radioactive compound. The closest method is the method of producing phenylacetone by reacting phenylacetic acid with acetic acid in the gas phase on a catalyst at a process temperature of 470 ° C. The initial mixture consists of phenylacetic acid - 2 vol.%, Acetic acid - 20 vol.%, Nitrogen as inert - 78 vol.%. The catalyst was a mixture of magnesium oxide with antimony oxide in a ratio of 1: 0.1, respectively. The catalyst was used in tablet form or was applied onto a spherical or cylindrical support. The yield of phenylacetone is 68.2%.
The disadvantage of the prototype is the low yield of phenylacetone, the use of a large excess of acetic acid, the need to dilute the mixture of starting acids with a large amount of nitrogen. The purpose of this invention is to increase yield, reduce production waste, simplify the technology for producing phenylacetone. This goal is achieved through the use of a method for producing phenylacetone by reacting phenylacetic acid with acetic acid in the gas phase on a catalyst at a temperature not lower than 350 ° C (the decomposition temperature of the resulting phenylacetic acetate), while cobalt oxide CoO is used as a catalyst (mol. 75 g / mol) on an inert carrier.
The mechanism for producing phenylacetone from a mixture of phenylacetic and acetic acid in the gas phase is reduced to the formation of phenylacetic acetate, which is an undetectable intermediate, which decomposes under the influence of temperature into phenylacetone and carbonate of the corresponding metal (depending on the type of catalyst). This factor determines the temperature at which the process. This statement is not justified. In the present invention, cobalt oxide was used as a catalyst in which the temperature for the decomposition of carbonate to oxide is 910 ° C, and yet the phenylacetone production reaction proceeds, with yields exceeding those in the above-described patent. From this it follows that the process can also take place when only metal carbonate is present as a catalyst. Cobalt oxide showed a higher activity than the catalysts described in the above patents. The method of derivatography was used to study the decomposition of acetates formed in the process. It was noted that symmetric cobalt acetates (acetic and phenylacetic) have the closest decomposition temperatures with respect to other types of catalysts, i.e. these substances are similar in their basicity. Probably, this factor also affected mixed phenylacetic acetate, allowing phenylacetone to form with respect to acetone and dibenzylketone.
In any of the above processes, and in the claimed one, an excess of acetic acid relative to phenylacetic acid is used. This is due to the fact that, according to the mechanism described above, dibenzyl ketone is also formed, which is formed from two phenylacetic acid molecules. Phenylacetic acid is a much more expensive reagent than acetic acid, and on this basis, in order to minimize the formation of dibenzylketone, an excess of acetic acid is used. In our case, for economic reasons (a large excess of acetic acid significantly increases the amount of formed acetone and carbon dioxide, thereby burdening the technological scheme with a more complex system for capturing phenylacetone and acetone, and reduces the productivity of the plant), we took a 3.5 molar excess of acetic acid, which sufficiently extinguished the dibenzylketone formation reaction without burdening the process with a large number of by-products.