The study also includes an elaborate discussion on the future potential of this evolving market. Amongst other elements, the report features:
Key Inclusions
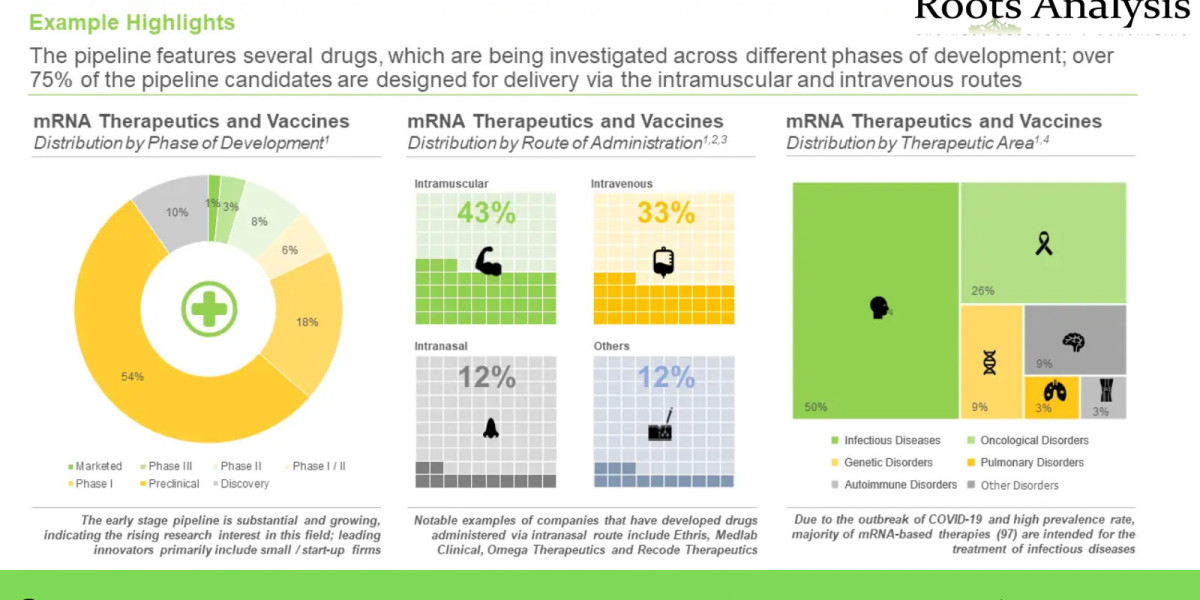
- A detailed overview of the overall market landscape of mRNA therapeutics and vaccines, based on several relevant parameters, such as phase of development (marketed, phase III, phase II, phase I / II, phase I, preclinical and discovery), type of drug candidate (mRNA therapeutic and mRNA vaccine), therapeutic area (infectious diseases, oncological disorders, genetic disorders, pulmonary disorders, autoimmune disorders and other disorders), type of delivery system used (lipid nanoparticles, lipopolyplex, liposomes, direct transfer, self-amplifying RNA platform and others), type of molecule encoded (spike protein, glycoprotein, antigen, cytokine, protein coding gene, tumor neoantigen, enzyme, tumor antigen and others) and route of administration (intramuscular, intravenous, intranasal and others).
- A detailed overview of the current mRNA therapeutics and mRNA vaccine market of players engaged in the development of mRNA therapeutics and vaccines, along with information on their year of establishment, company size (in terms of number of employees), location of headquarters, regional landscape and key players engaged in this domain.
- An in-depth company competitiveness analysis of mRNA therapeutics and vaccines developers based on their portfolio strength (in terms of year of establishment and company size), pipeline strength (in terms of its pipeline maturity and drug administration route) and number of therapeutic areas targeted.
- Detailed profiles of marketed and late stage (phase III) mRNA therapeutics and vaccines, along with information on the development timeline of the therapy, current development status, primary target indication, therapeutic area, type of molecule encoded, type of delivery system used, affiliated technology, recent partnerships associated with the drug, clinical trial results related to the drug and other recent developmental.
- A review of the various mRNA-focused initiatives undertaken by big pharma players (shortlisted on the basis of the revenues generated in 2021), featuring a [A] heat map representation that highlights mRNA therapeutics and vaccines under development (in partnership with core mRNA-focused entities), along with information on funding amount raised, partnership activity, and diversity of product portfolio (in terms of disease indication(s) being treated and focus therapeutic area(s)), as well as [B] a spider web representation, comparing the initiatives of big pharmaceutical players on the basis of multiple relevant parameters.
- An analysis of start-ups (established between 2016-2022) engaged in this domain, based on several relevant parameters, such as number of candidates in discovery, preclinical and clinical phases of development, amount raised, number of investors and number of deals inked.
- An analysis of completed, ongoing and planned clinical studies of mRNA therapeutics and vaccines, based on several relevant parameters, such as trial registration year, trial status, trial phase, target therapeutic area, study design, type of sponsor / collaborator, leading industry players (in terms of number of trials conducted), enrolled patient population and key geographical regions.
- An analysis of recent collaborations and partnerships within the mRNA therapeutics and vaccines industry, based on several relevant parameters, such as year of partnership, type of partnership, targeted therapeutic area, most active players (in terms of number of deals inked) and regional distribution of partnership activity that have been undertaken in this domain, during the period 2013-2022.
- A detailed analysis of various investments made by players engaged in this domain, during the period 2013-2022, based on several relevant parameters, such as year of funding, type of funding (seed financing, venture capital, IPOs, secondary offerings, debt, grants and other offerings), amount invested, therapeutic area, most active players (in terms of number of funding instances and amount invested) and key investors (in terms of number of funding instances).
- An in-depth analysis of the various patents that have been filed / granted related to mRNA therapeutics and vaccines domain, since 2016, based on several relevant parameters, such as type of patent (granted patents, patent applications and others), publication year, geographical region, CPC symbols, leading industry players (in terms of the number of patents filed / granted) and patent valuation.
The report also features the likely distribution of the current and forecasted opportunity across important market segments, mentioned below:
- Routes of Administration
- Intradermal
- Intravenous
- Intranasal
- Others
- Therapeutic Area
- Infectious Diseases
- Oncological Disorders
- Other Disorders
- Key Geographical Regions
- North America
- Europe
- Asia-Pacific
- Latin America
- Middle East and North Africa
Key Questions Answered
- Which are the key players engaged in the development of mRNA therapeutics and vaccines?
- Which mRNA drug candidates are being evaluated across early and late stages of clinical development?
- Which are the key therapeutic areas targeted by mRNA therapeutics and vaccines?
- Which type of delivery systems are primarily being used for the delivery of mRNA therapeutics and vaccines?
- Which are the most active trial sites (in terms of number of clinical studies being conducted) related to mRNA therapeutics and vaccines?
- Which companies are actively filing patents in order to drive innovation in the field of mRNA therapeutics and vaccines?
- What type of partnership models are commonly adopted by stakeholders in the mRNA therapeutics and vaccine domain?
- Which are the companies that are investing heavily in this domain?
- How is the current and future market opportunity likely to be distributed across the various key market segments?
To view more details on this report, click on the link
https://www.rootsanalysis.com/reports/mrna-therapeutics-and-vaccines-market.html
News article
Modular Facility Manufacturers Market
Learn from experts: do you know about these emerging industry trends?
Novel Cell Cytometers: Need of the Hour
Antiviral Drugs: The Unmet Requirement in the Pharmaceutical Space
About Roots Analysis
Roots Analysis is a global leader in the pharma / biotech market research. Having worked with over 750 clients worldwide, including Fortune 500 companies, start-ups, academia, venture capitalists and strategic investors for more than a decade, we offer a highly analytical / data-driven perspective to a network of over 450,000 senior industry stakeholders looking for credible market insights.
Learn more about Roots Analysis consulting services:
Roots Analysis Consulting - the preferred research partner for global firms
Contact:
Ben Johnson
+1 (415) 800 3415