Roots Analysis has announced the addition of “Bioavailability Enhancement Technologies and Services Market (2nd Edition), 2022-2035” report to its list of offerings
Considering the existing drug solubility and biocompatibility-related concerns and their impact on drug safety and patient compliance, the demand for bioavailability enhancement solutions has gained significant traction from the medical community. Drug developers have demonstrated a preference to continue relying on bioavailability enhancement service and technology providers, in order to optimize drug development cost and research timelines. Furthermore, driven by the large number of BCS class II and IV therapies in the current pipeline and growing demand for effective therapeutics, the bioavailability enhancement services market is expected to grow at a steady pace in the foreseen future.
Key Market Insights
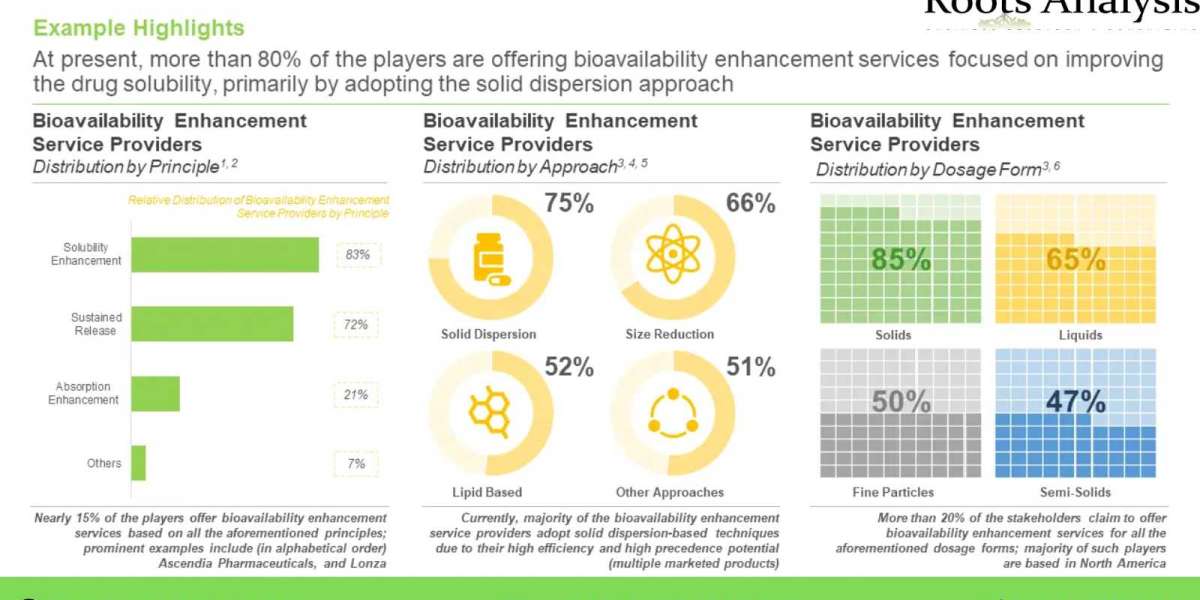
~115 players currently claim to be engaged in providing bioavailability enhancement services
The bioavailability enhancement services market landscape is primarily characterized by the presence of small, mid-sized and large firms, which collectively constitute over 75% of the total share. Further, 45% of the service providers are based in North America, followed those having headquarters in Europe and Asia.
More than 75 bioavailability enhancement technologies are presently available in the market
Most (55%) of these technologies support solubility enhancement, followed by those providing support for sustained release drug delivery (46%). Additionally, nearly 70% of the technologies provide bioavailability enhancement services for solids, followed by liquids and fine particles / powders.
Partnership activity in this field has increased at a CAGR of nearly 35%, between 2013 and 2022
Mergers and acquisitions (~40%) emerged as the most common type of partnership models adopted by stakeholders engaged in this industry. Nearly 55% of the total deals were established post-2018, with the maximum activity being reported in 2021.
9,600 patents related to bioavailability enhancement have been filed / granted, during 2003-2022
Majority (~80%) of the patents were filed as applications, followed by granted patents (20%). It is important to highlight that, nearly 1,200 patents were filed / granted in the year 2021. Further, 36% of the total patents were filed in North America, indicating the extensive RD activity in this region.
4,700+ clinical trials evaluating bioavailability of drug candidates have been registered worldwide
Clinical research activity, in terms of number of trials registered, is reported to have increased at a CAGR of 24%, during the period 2010-2022. Of the total, more than 85% of the studies have already been completed, followed by active trials that are actively recruiting patients (~10%).
Technology evaluation framework provides a value addition matrix for bioavailability enhancement approaches currently employed by stakeholders
The framework highlights the implementation of several advanced as well as traditional bioavailability enhancement approaches and technologies at different stages of the drug development pathway. Further, it provides a detailed analysis on ease of implementation and associated risk in integrating such technologies, based on various parameters, such as number of technologies, number of approved drugs, trends highlighted in published literature and patents, and business models adopted by industry stakeholders.
Shifting focus of drug developers towards development of lipophilic drug compounds is anticipated to drive the demand for bioavailability enhancement technologies and services in the next 13 years
The outsourced commercial demand for bioavailability enhancement is projected to increase at a CAGR of ~10 %. Further, the current clinical demand for solid dosage form captures around 30% of the total demand.
North America and Europe are anticipated to capture more than 65% of the market share, by 2035
Owing to the high prevalence of diseases, increasing investments in formulation development research, and growing initiatives for drug discovery and personalized medicine in Asia, the bioavailability enhancement services market in Asia is likely to grow at a relatively faster pace (13.54%) in the long term. Further, in 2035, bioavailability enhancement services for new chemical entities are expected to dominate the market, capturing around 85% of the market share.
To request a sample copy / brochure of this report, please visit
https://www.rootsanalysis.com/reports/198/request-sample.html
Key Questions Answered
- Who are the key players engaged in the bioavailability enhancement technologies and services market?
- Which are the key geographies where bioavailability enhancement technology and service providers are located?
- What are the recent developments and expected trends in the bioavailability enhancement industry?
- Which partnership models are commonly adopted by stakeholders offering bioavailability enhancement solutions?
- What is the evolving trend of publications focused on bioavailability enhancement technologies?
- Which companies are actively filing patents to drive innovation in the bioavailability enhancement market?
- What are the key market trends and driving factors that are likely to impact the growth of the bioavailability enhancement services market?
- How is the current and future opportunity likely to be distributed across key market segment?
The financial opportunity within the bioavailability enhancement services market has been analyzed across the following segments:
- Drug Class
- New Drug Approvals
- Generics
- BCS Classification
- BCS II Drugs
- BCS IV Drugs
- Bioavailability Enhancement Approach
- Solid Dispersion
- Size Reduction
- Lipid-based
- Dosage Form
- Liquids
- Solids
- Semi-Solids
- Fine Particles / Powders
- Key Geographies
- North America
- Europe
- Asia
- Latin America
- Middle East and North Africa (MENA)
- Rest of the World
The research includes profiles of key players (listed below); each profile features a brief overview of the company, details related to its financial information (if available), recent developments (including partnerships and collaborations) and an informed future outlook.
- Adare Pharma Solutions
- Ascendia Pharmaceuticals
- Catalent
- Lonza
- Lubrizol Life Science Health
- Pace Life Sciences
- Quotient Sciences
- WuXi STA (a subsidiary of WuXi AppTec)
For additional details, please visit
You may also be interested in the following titles:
- Smart Labels Market: Industry Trends and Global Forecasts, 2022-2035
- 4D Bioprinting Market : Industry Trends and Global Forecasts, 2022-2035
About Roots Analysis
Roots Analysis is a global leader in the pharma / biotech market research. Having worked with over 750 clients worldwide, including Fortune 500 companies, start-ups, academia, venture capitalists and strategic investors for more than a decade, we offer a highly analytical / data-driven perspective to a network of over 450,000 senior industry stakeholders looking for credible market insights.
Contact:
Ben Johnson
+1 (415) 800 3415
Ben.johnson@rootsanalysis.com